Unveiling the Chemistry of HCOOCH CH2 H2O: Structure, Reactions, and Industrial Relevance
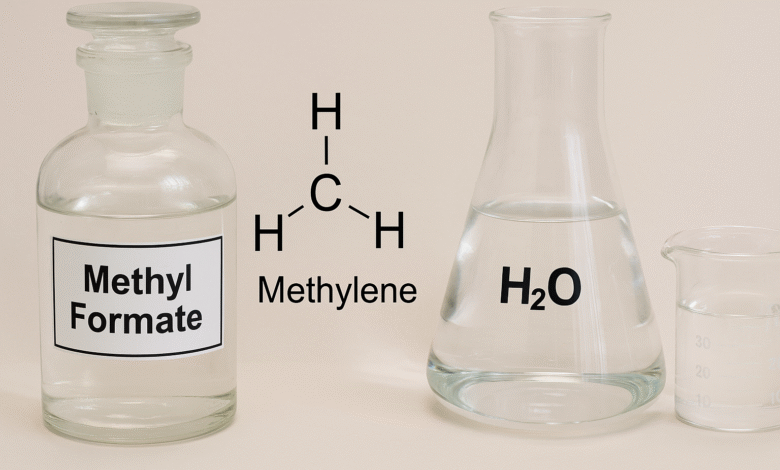
In the realm of organic and industrial chemistry, certain molecular combinations offer significant insight into reaction mechanisms and real-world applications. Among these is hcooch ch2 h2o—a trio comprising methyl formate (HCOOCH), a methylene group (CH2), and water (H2O). While simple in their individual identities, their interactions and collective behavior form a critical basis for multiple chemical processes and commercial technologies.
What is HCOOCH CH2 H2O?
To appreciate the value of hcooch ch2 h2o, it’s necessary to understand its components in detail:
-
HCOOCH (Methyl Formate): A common ester of formic acid, methyl formate plays a role in fragrance production, solvents, and chemical intermediates.
-
CH2 (Methylene Group): This reactive unit is not a stable molecule on its own but often appears in various organic compounds, facilitating carbon-carbon bonding.
-
H2O (Water): Known as the universal solvent, water is more than just a medium—it’s often a direct participant in many reactions.
The combination of hcooch ch2 h2o can appear in synthetic chemistry, esterification, hydrolysis, and in energy systems such as fuel cells.
Chemical Roles of HCOOCH CH2 H2O
Methyl Formate: A Reactive Ester
HCOOCH, or methyl formate, is a low-boiling ester formed by the reaction of formic acid and methanol. It is commonly used as an intermediate in organic synthesis. In systems involving hcooch ch2 h2o, this compound frequently acts as a starting point for further chemical transformations, especially those involving methylene bridges or hydrolysis reactions.
CH2: The Link Between Structures
Though CH2 is rarely seen on its own, it represents the methylene bridge that connects functional groups in organic molecules. In hcooch ch2 h2o reactions, the CH2 unit can come from formaldehyde, methanol, or related compounds, enabling transformations like polymerization or alcohol synthesis.
Water: More Than a Solvent
Water’s role in hcooch ch2 h2o interactions goes far beyond being just a solvent. It often actively participates in hydrolysis and equilibrium reactions. In esterification or breakdown reactions, water directly affects the speed and outcome of these processes.
Industrial and Laboratory Applications of HCOOCH CH2 H2O
Chemical Synthesis and Solvent Systems
The combination of hcooch ch2 h2o is frequently observed in chemical production processes. Methyl formate serves as both a reactant and a solvent in synthesis routes for pharmaceuticals, perfumes, and resins. The presence of water and methylene intermediates plays a significant role in directing the pathway and efficiency of these reactions.
Energy Applications
Formic acid derivatives like methyl formate are being explored for hydrogen storage and fuel cell technology. In aqueous systems, hcooch ch2 h2o may act as a controlled-release hydrogen source. CH2 groups facilitate energy transfer and storage, enhancing the practical use of this trio in next-generation energy solutions.
Polymer and Resin Production
Through reactions involving CH2 bridges, hcooch ch2 h2o becomes relevant in the production of polymers and synthetic resins. These applications use the methylene group for cross-linking, while water ensures the proper medium for polymerization.
Common Reactions Involving HCOOCH CH2 H2O
Esterification and Hydrolysis
A well-known reaction involving components of hcooch ch2 h2o is the formation of methyl formate:
HCOOH + CH3OH ⇌ HCOOCH3 + H2O
This reversible esterification process highlights the dynamic interplay among formic acid, alcohols, and water. Methyl formate can also be hydrolyzed back to formic acid and methanol in the presence of water.
Formaldehyde Production
Under thermal or catalytic conditions, methyl formate can decompose to generate formaldehyde (HCHO), a molecule featuring the methylene group. Water once again acts as a moderator, controlling reaction speed and temperature stability.
Safety and Environmental Concerns
Handling of Methyl Formate and Related Compounds
Methyl formate is flammable and volatile, requiring careful handling in both industrial and laboratory environments. Any application involving hcooch ch2 h2o should adhere to strict safety protocols, including the use of personal protective equipment and proper ventilation.
Environmental Impact and Waste Management
Compounds involved in hcooch ch2 h2o systems may emit volatile organic compounds (VOCs). Safe disposal and adherence to environmental guidelines are critical to avoid contamination of water and air resources. Green chemistry approaches aim to minimize such hazards by employing bio-based raw materials and recyclable catalysts.
Laboratory Utility of HCOOCH CH2 H2O
As Reaction Media
In research labs, hcooch ch2 h2o combinations are often chosen for their balanced reactivity and solvent properties. Methyl formate mixed with water and methylene-based additives forms a reliable medium for conducting titrations, extractions, or syntheses.
Analytical Chemistry
The unique physical properties of hcooch ch2 h2o affect parameters like pH, polarity, and volatility. These characteristics make the trio suitable for analytical methods such as gas chromatography, spectroscopy, and electrochemical analysis.
Future Outlook and Research Trends
With increasing attention on sustainability, hcooch ch2 h2o is gaining traction in:
-
Bio-based Chemistry: Developing chemical routes using renewable sources like plant-derived formic acid or methanol.
-
Catalyst Innovation: Designing catalytic systems that make reactions involving hcooch ch2 h2o faster and cleaner.
-
Green Fuels: Utilizing aqueous formate solutions in compact and portable fuel cells.
These areas suggest promising avenues for innovation using this simple yet powerful chemical combination.
FAQs
Q1: What is the significance of hcooch ch2 h2o in chemical reactions?
A: This trio plays a central role in esterification, hydrolysis, and energy-transfer processes. It represents the interaction of a reactive ester (methyl formate), methylene intermediates, and water.
Q2: Is hcooch ch2 h2o safe to handle in laboratory conditions?
A: With proper safety measures—gloves, goggles, and ventilation—hcooch ch2 h2o systems can be safely handled. Methyl formate, however, is flammable and must be used with caution.
Q3: Can hcooch ch2 h2o be used in green chemistry?
A: Yes, the trio fits well within sustainable chemistry frameworks, especially when derived from biomass or paired with recyclable catalysts.
Q4: How does water influence reactions involving hcooch ch2 h2o?
A: Water can act both as a solvent and a reactant, influencing reaction kinetics, pH levels, and product yields.
Q5: Where is hcooch ch2 h2o used industrially?
A: Industries utilize this combination in fragrance synthesis, fuel cell technologies, polymer production, and as a chemical intermediate.
Read also:The Mystery of Rowdy Oxford Integris: What’s Behind the Hype?
Why “Lake Texoma Should Be Capitalized”: A Complete Writing Guide
Understanding Every Type of FOK959S-M: A Comprehensive Guide for Modern Tech Users